How To Find Atomic Number Of An Element
Diminutive Number and Mass Numbers
After reading this section y'all will be able to do the following:
- Define and determine the atomic number of an atom.
- Ascertain and decide the mass number of an atom.
Atomic Number
The number of protons in the nucleus of an atom determines an chemical element'southward atomic number. In other words, each element has a unique number that identifies how many protons are in 1 atom of that element. For example, all hydrogen atoms, and only hydrogen atoms, contain ane proton and have an diminutive number of 1. All carbon atoms, and only carbon atoms, contain six protons and have an atomic number of 6. Oxygen atoms comprise viii protons and take an atomic number of 8. The diminutive number of an chemical element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same.
Isotopes are forms of elements that take the aforementioned number of protons and therefore the same atomic number, simply a unlike number of neutrons which affects their mass number.
Mass Number
All atoms have a mass number which is derived every bit follows:
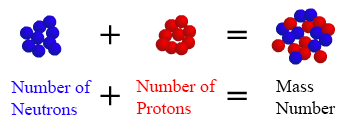
In words, the mass number is the number of neutrons in an atom of a specific element plus the number of protons in an atom of that chemical element. For instance the mass number of a regular carbon cantlet is 12, since a carbon atom has six protons and 6 neutrons in its nuclus. The mass number is approximately equal to the atomic mass,which is the mass of a single cantlet of a chemical element measured in atomic mass units (amu). The atomic mass of Hydrogen is 1.00797 amu and the atomic mass of Carbon is 12.011 amu. The atomic mass is typically listed in the periodic tabular array below the chemical element's proper name.
Since isotopes take a different number of neutrons, their mass numbers and diminutive masses differ from those listed in the periodic table. Isotopes are discussed more in depth later on in this module.
Review:
- An element'southward or isotope'south diminutive number tells how many protons are in its atoms.
- An element's or isotope's mass number tells how many protons and neutrons in its atoms.
Source: https://www.nde-ed.org/Physics/AtomElements/atomicmassnumber.xhtml
Posted by: alfordtheyetage.blogspot.com


0 Response to "How To Find Atomic Number Of An Element"
Post a Comment